In our brain, there are around 10 billion neurons and the neural synapses are the basic and connection units transferring the information between them. The neural transmitters, released by presynaptic neurons, diffuse into the synaptic cleft and then bind to the corresponding receptors on postsynaptic neurons, thereby regulating their neuronal activity and accomplishing the information transmission. Any deregulation of this process is regarded as one of the most leading causes for the neurological disorders.
Glutamate is the major excitatory neurotransmitter in the brain, and the postsynaptic expression level of glutamate receptors is a critical factor to determine the efficiency of information transmission and the activity of neuronal network. Therefore, glutamate receptors trafficking is a critical basis for the physiological function of our brain circuit.
It is known that KAR-type glutamate receptors are closely related to many neurological disorders. Dr. SHENG Nengyin from Kunming Institute of Zoology, Chinese Academy of Sciences has long been devoted to studying the regulation of KARs trafficking and achieved many important findings. His previous studies (Elife 2015; JBC 2017; PNAS 2017) have revealed that the trafficking performance of KAR subunits GluK1 and GluK2 are totally different in neurons, and it is the amino acid sequences of their extracelluar domains determining their distinct trafficking capability.
To resolve the molecular machinery, the “Neural Synaptic Mechanism and Function” group leaded by Dr. SHENG Nengyin, collaborated with Dr. SHI Yun Lab from Model Animal Research Center, Nanjing University. The researchers made serial chimeric GluK1-GluK2 receptors based on their conserved structures. Applying electrophysiological technique on hippocampal slice cultures to analyze the synaptic responses mediated by these receptors, the researchers unexpectedly found a crucial inhibitory role of the signal peptide for GluK1 trafficking. When the signal peptide of GluK1 was replaced by that of GluK2, the GluK2 signal peptide reversed the incapability of synaptic trafficking. The resultant GluK1(SPGluK2) receptor potentiated the synaptic responses similar as wild-type GluK2, and this potentiation was suppressed by coexpressed GluK1 signal peptide in the same neurons.
Furthermore, the researchers found that the inhibitory function of GluK1 signal peptide requires the presence of the N-terminal domain, as replacing it with the corresponding sequences of GluK2, GluK1 would traffic to synapses and neuronal surface successfully. With biochemical studies, they found GluK1 signal peptide directly interacts with its N-terminal domain (ATD). Therefore, they propose a model that in a trans manner and behaving as ligand of GluK1, the cleaved signal peptide binds to GluK1 ATD and forms an inhibitory complex regulating GluK1 forward trafficking in neurons.
Signal peptides are N-terminal residues of newly synthesized secretory or membrane proteins. Generally, they are regarded as ‘cellular address codes’ for intracellular trafficking, localization and secretion. This study has revealed a significant function of signal peptide for glutamate receptor trafficking and uncovered a novel trafficking mechanism for glutamate receptors. This might provide important theoretical basis to further study synaptic transmission and plasticity, as well as the pathological mechanism of related neurological disorders. The results have been published online on the journal of Nature Communications with the title of “Signal peptide represses GluK1 surface and synaptic trafficking through binding to amino-terminal domain”, on **, 2018. Dr. SHENG Nengyin is the leading co-corresponding author, together with Dr. SHI Yun. This work was funded by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences, National Natural Science Foundation of China, Ministry of Science and Technology of China and the Chinese Academy of Sciences Pioneer Hundred Talents Program.
Link to the paper: https://www.nature.com/articles/s41467-018-07403-7
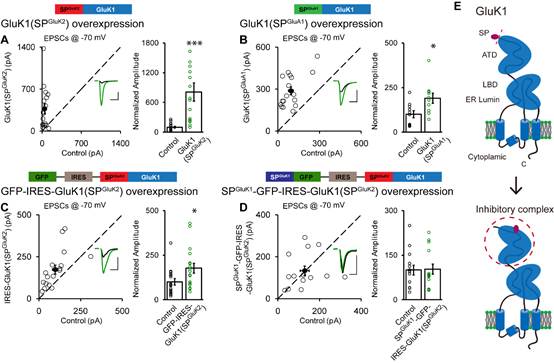
Working Model of the inhibitory complex of GluK1 postcleavage signal peptide and ATD (E) and the presentative electrophysiological recording results suggesting the trans-inhibition by GluK1 signal peptide. (Image by Dr. SHENG Nengyin’s and Dr. SHI Yun’s Group)
By SHENG Nengyin, Editor: HE Linxi
Contact:
HE Linxi
helinxi@mail.kiz.ac.cn
