Mitogenome evidence disclosed Ice Age Human migrations from northern coastal China to the Americas and Japan
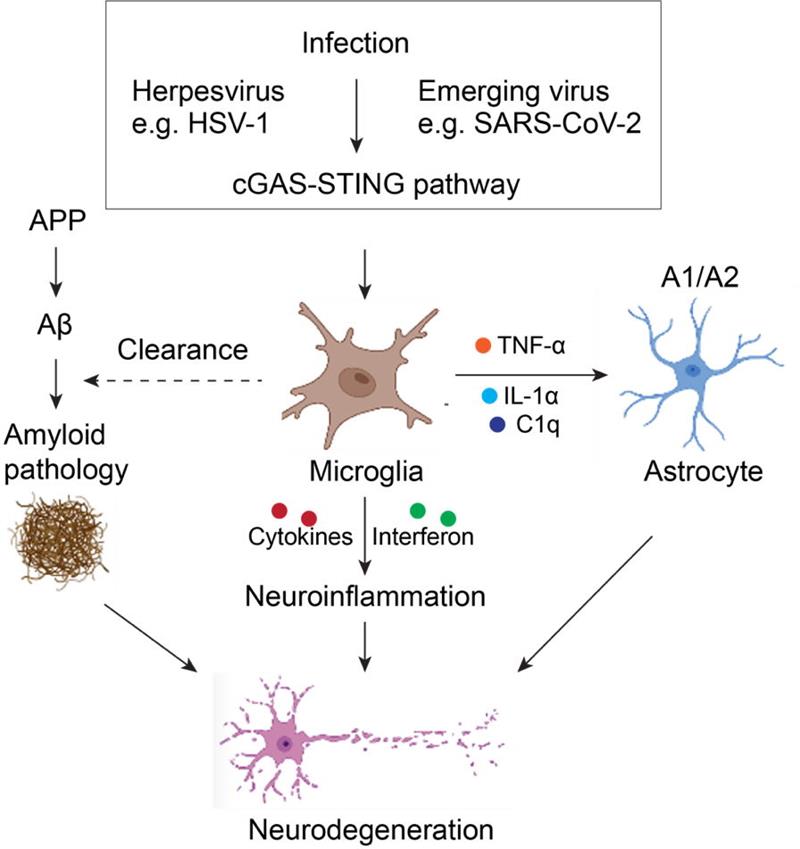
LERAN MOREA research lab led by Dr. ZENG Jianxiong from Kunming Institute of Zoology of the Chinese Academy of Sciences, in collaboration with Prof. ZHAO Zhen from Keck School of Medicine, University of Southern California, have discovered the cGAS-STING pathway-mediated innate immune mechanism underlying the development and progression of neurodegenerative Alzheimer's disease (AD).
AD mainly occurs in the elderly population. Its typical symptoms include progressive memory loss and cognitive dysfunction associated with characteristic amyloid plaque, neurofibrillary tangle, and neuronal loss in the afflicted individual's brain. Unfortunately, no effective therapeutic drugs are currently available, calling for further clarifying the new pathogenic mechanism underlying AD.
Triggers, including pathogen infection, have been known to be important risk factors for AD. Accordingly, previous studies have shown that these risk factors lead to abnormal accumulation of cytosolic nucleic acid from pathogens and hosts, which has the potential to induce innate immune responses in cells. However, the role of accumulated nucleic acid in the cytoplasm and resulted neural innate immunity in the development and progression of AD remains poorly known.
Cyclic GMP-AMP synthase (cGAS) is an important nucleic acid sensor in the cytoplasm, which has emerged as a critical mechanism for coupling the sensing of DNA, the binding of cGAS to double-stranded DNA (dsDNA). The binding event allosterically activates cGAS catalytic activity and leads to the production of 2′3′ cyclic GMP–AMP (2′3′-cGAMP), a second messenger molecule and potent agonist of stimulator of interferon genes (STING). This biochemical cascade eventually induces downstream transcription factors IRF3 and NF-κB mediated production of type I interferon and a variety of inflammatory factors, triggering innate immune response program.
Previous studies have shown that infection with viral pathogens such as herpes simplex virus type I (HSV-1) induces the activation of the cGAS-STING pathway. In this study, researchers cultured multiple types of primary neural cells and confirmed that the activation of the cGAS-STING pathway occurred mainly in microglia, the resident immune cells in the central nervous system.
Further, the cGAS-STING pathway is activated in the brain of human patients with AD. The activation of the cGAS-STING pathway had a significant effect on neuroinflammation, microglial phagocytosis, and astrocytic subtyping via Cgas deficiency in 5×FAD mice, a model of AD amyloid pathology. Furthermore, it was demonstrated that a STING inhibitor could suppress neuroinflammatory responses and alleviate AD pathogenesis in 5×FAD mice.
In summary, researchers not only revealed the critical role of the cGAS-STING pathway in the development and progression of AD, offering a potential targeting for precise AD intervention, but also a new neural innate immune mechanism underly neurodegeneration induced by pathogen infection.
This study, entitled “Activation of innate immune cGAS-STING pathway contributes to Alzheimer’s pathogenesis in5×FAD mice” was published in Nature Aging. Meanwhile, a research briefing titled “Microglial cGAS-STING links innate immunity and Alzheimer's disease”, commenting on the innovative findings in the paper, was accompanied for publication. Dr. ZENG Jianxiong and Prof. ZHAO Zhen are the co-corresponding authors. XIE Xiaochun, MA Guanqin, and LI Xiaohong are the co-first authors.
This project was supported by the Yunnan Fundamental Research Projects.
Graphic abstract:
Contact:
Dr. ZENG Jianxiong, Principal Investigator
Kunming Institute of Zoology, Chinese Academy of Sciences,
Kunming, Yunnan 650201, China
Tel: 86-871-65176629
E-mail: zengjianxiong@mail.kiz.ac.cn