A research lab led by Prof. ZHENG Ping from Kunming Institute of Zoology of the Chinese Academy of Sciences has identified a p53-independent quality control factor which favors the rapid shutdown of the regulatory circuitry of pluripotency at the post-transcriptional levels, thereby ensuring robust differentiation and apoptosis in response to unrepaired DNA damage.
ESCs hold wide applications in biomedical researches and in cell-based regenerative medicine as their properties of unlimited proliferation and differentiation into all cell types in the body. However, genomic instability and tumorigenicity hamper their full applications. Thus, elucidating the underlying mechanism is an essential prerequisite for their clinical applications.
It is interesting to note that ESCs have super competence to maintain stable genome. For instance, mouse ESCs display 100-fold lower mutation rates than their isogenic embryonic fibroblasts. Although the underpinning mechanism remains largely unknown, previous studies show that ESCs can undergo rapid apoptosis or differentiation to eliminate cells with unrepaired DNA damage and prevent DNA damage from spreading onto progenies.
Tumor suppressor protein TRP53 (also known as p53) plays a crucial role in above process. Mechanically, p53 switches off pluripotency at the transcriptional level to safeguard ESCs’ quality. However, ESCs pluripotency state is not only regulated at the transcriptional level but but also at post-transcriptional level. For instance, protein and transcript levels usually become decoupled at the early phase of dynamic cell fate transition, suggesting a vital role of the post-transcriptional regulation in initiating cell fate transition. On the basis of above knowledge, there might be p53-independent posttranscriptional mechanism to facilitate the rapid pluripotency switching off in response to DNA damage.
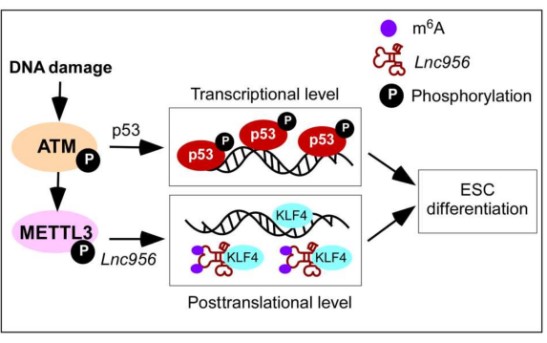
In this study, researchers reveal a post-translational mechanism which acts downstream of Ataxia telangiectasia mutated (ATM) kinase but is parallel to the p53-mediated transcriptional pathway. This post-translational regulation is mediated by an ESC-specific lncRNA NONMMUT028956 (Lnc956 for short). Upon DNA damage, Lnc956 undergoes m6A methylation that drives Lnc956 to interact with KLF4. Lnc956-KLF4 association sequestrates the KLF4 protein and disrupts its transcriptional regulations on pluripotency, thereby favoring the rapid shutdown of the regulatory circuitry of pluripotency before the protein levels of the core pluripotent transcription factors go decline.
In summary, researchers uncover a p53-independent quality control factor which ensures robust differentiation and apoptosis to eliminate damaged cells and maintain genome stability of the cell population.
This work entitled " Lnc956 regulates mouse embryonic stem cell differentiation in response to DNA damage in a p53-independent pathway " was recently published in Science Advances. Prof. ZHENG Ping and MA Huaixiao are the co-corresponding authors. MA Huaixiao, NING Yuqi and WANG Lin are the co-first authors.
This work was supported by National Natural Science Foundation of China (31930027), National Key Research & Developmental Program of China (2021YFA1102002), and Yunnan Fundamental Research Projects (2021000051 and 202201AS070041).
Graphic abstract:
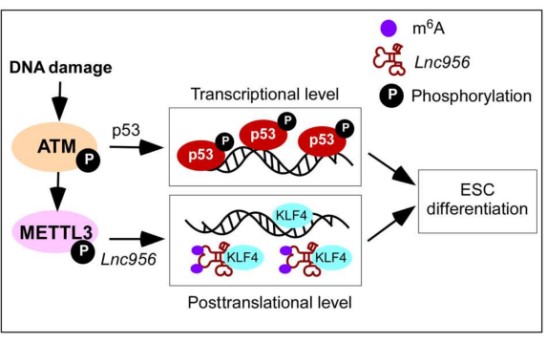
Working model for quality control of ESCs. Downstream of ATM activation, p53 pathway, and Lnc956-KLF4 axis work independently to ensure robust elimination of ESC population with unrepaired DNA damages. (Image by ZHENG Ping)
(By ZHENG Ping)
Contact:
Prof. ZHENG Ping, Principal Investigator
Kunming Institute of Zoology, Chinese Academy of Sciences,
Kunming, Yunnan 650201, China
Tel: +86 871 65197853
E-mail: zhengp@mail.kiz.ac.cn