The latest research on Major histocompatability complex class I (MHC I) from Dr. ZHENG Yongtang’s team at the Kunming Institute of Zoology, CAS, perfectly illustrates the idea that even on a cellular level, two is better than one. Prof. ZHENG’s team examined how MHC I molecules and some of its specific variants interact to form unique structures that prolong the life of the cell and promote the strength and stability of specific proteins by preventing cell ubiquitation, thereby delaying protein recycling.
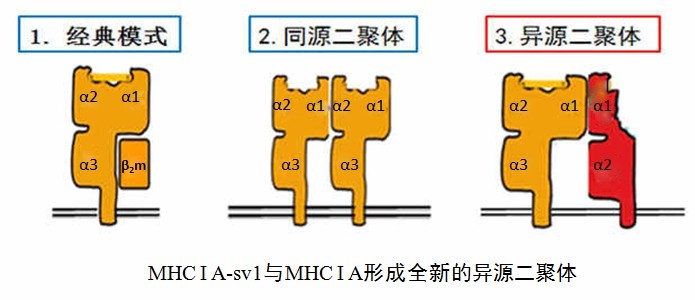
MHC I molecules play a pivotal role in the immune recognition of intracellular pathogens by presenting antigenic peptides to CTLs and by regulating cytolytic activities of NK cells. In mice, humans and non-human primates, a number of important splice variants of these molecules have been observed. Some of these variants, often called “open conformers” seem to come specifically from mature MCH I proteins rather than simply protein misfolding. These in turn form homodimers and other cell receptors on the surface of the cell, which are speculated to enhance Ag recognition by CD8+ T cells.
Other studies have noted that like many other immunological relevant genes, MHC I transcripts were reported to undergo alternative splicing, demonstrating that this takes place, even at a small level, in nearly all cells and may be a common phenomenon across numerous different species. Despite this shared characteristic, until Dr. ZHENG’s research, little was known about how the MHC I variants regulate and work with full MHC I molecules. Using biological and biochemical methods, Dr. ZHENG’s team demonstrated that in the rhesus macaques there is a specific MHC I A splice variant, named “MHC I A-sv1”. The MHC I A-sv1 is expressed on the cell surface as a long-lived immature glycoprotein that can self-interact or associate with a fully mature MHC I A molecule, forming heterodimers. Both structures lack the β2m molecule. Moreover, the heterodimeric complex inhibits the ubiquitination of the full-length MHC I A protein, prolonging its cell surface turnover time. The experiments demonstrated that the coexpression of both the MHC I A and MHC I A-sv1 work in tandem to drastically prolong the life of the cell, as opposed to when only MHC I A is expressed. This research and others suggest that the coexpression of both proteins changes the turnover kinetics to protect MHC I A from cellular degradation.
For now, precisely how the heterodimeric structure prevents ubiquitation of the MHC I A is not known, but it seems likely that the heterodimer causes a conformational change that keeps the cell from beginning ubiquitination, or that the lack of the b2m in MHC I A heterodimer alters the interaction with the E3 ubiquitation ligase. Future research will hopefully shed more light on the precise mechanics of this interaction. Likewise, the results of the study lead to several further questions, notably how the heterodimer between MHC I A-sv1 and MHC I A plays out in MHC I Ag Presentation and how the heterodimeric complex functions the strength of interactions between MHC I A and KIR on the NK cell surface. Further investigation of these possibilities may give insights into the functional regulation of the MHC I A splice variants in the immune response.
The data from this research demonstrates for the first time that the splice variant of MHC I A forms a unique heterodimeric structure with MHC I A, which reduces the ubiquitin-mediated degradation of MHC I A and consequently promotes the stability of MHC I A proteins. This also suggests that MHC I A-sv1 complicates the typical scenario of CTL and NK cell-mediated immune response by MHC I A molecules and demonstrate that the rhesus monkey may be an experimental model in which to further explore the functional significance of the membrane-bound classical MHC I splice variant in vivo.
This study has unveiled an important piece of new information regarding cis-regulation of MHC I A expression, which may have important functional implications at the level of trans-interactions. Dr. ZHENG’s research promises to break further ground towards fully understanding these interactions. For more, read the entire article in the Journal of Immunology: The β2-microglobulin–free heterodimerization of rhesus monkey MHC class I A with its normally spliced variant reduces the ubiquitin-dependent degradation of MHC class I A.
The main findings of this research have been published on Journal of Immunology, 2012, 188(5): 2285-2296. http://www.jimmunol.org/content/188/5/2285.abstract
