Drug addiction is a major social problem for worldwide. While drug usage is often detrimental to mental and physical wellbeing of the user, addiction frequently leaves family, friends and social programs with heavy burdens. Complicating this situation, drug addiction lacks a complete and effective treatment plan, as well as a high rate of relapse after withdrawal. Unfortunately, to date little is known about the biological mechanisms that underpin addiction, making it difficult to develop novel and effective treatment and intervention strategies.
Addiction itself is a chronic recurring disease marked by dependence on and uncontrollable cravings for a substance. While many studies have tried to explain risk factors for addiction, there is a relative lack of information on the underling biological mechanics that fuel it. One potential answer may lie in the cells that constitute living organisms. Mitochondria, which generate much of a cell’s energy, are closely related to metabolic processes, and consequently mitochondrial metabolic abnormalities are associated with a variety of degenerative diseases, especially those of the nervous system.
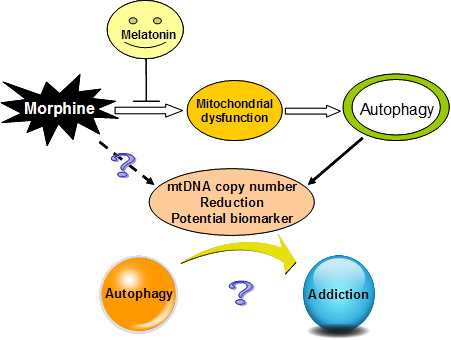
Building on the basic premises of what is currently known about mitochondria and cellular metabolism, under the careful guidance of Professors Yao Yong-Gang and Xu Lin, students at the Kunming Institute of Zoology (KIZ), Chinese Academy of Sciences (CAS)—Feng Yuemei, Jia Yunfang and Su Lingyan—carried out a series of studies that examined cells, animal models, and heroin addicted patients. They found that the mitochondrial copy number was much lower in morphine-treated nerve cells, hippocampal tissue and blood samples among morphine-addicted rats and heroin addicts. Further research showed that the morphine induced reduction in mitochondrial copy number was mediated by autophagy. Moreover, they also found that mitochondrial-targeted antioxidant melatonin treatment was able to restore mitochondria and prevent an further autophagy.
In establishing the rodent model of morphine addiction, Feng’s team found that pretreatment with low-doses of melatonin could block the morphine-induced behavioral sensitization and pain tolerance, and that the effect of melatonin on morphine induced hippocampal tissues was accompanied by reduced autophagy. At the same time, the mitochondrial DNA copy number returned to close to that in the control group. Feng’s group also noticed that during a 6 month follow-up of heroin addicts who had undergone detoxification, the melatonin levels in plasma increased relative to that at the beginning of detoxification, as did the mitochondrial DNA copy number; however, neither was restored to the observed level in a normal population.
Taken together, these results suggest that a decrease in mitochondrial DNA copy number and a reduction in plasma melatonin levels might serve as biomarkers for addiction, and that autophagy may provide a new perspective on the mechanisms of drug addiction. Hopefully future clinical and experimental studies can build on these findings and begin developing novel targeted treatments that will have more success in helping deal with the complex nature of addiction.
This study was recently published in the Journal of International Autophagy, available online at http://www.landesbioscience.com/journals/autophagy/article/25468/.
(By Andrew Willden)
