Apoptosis resistance is a hurdle for cancer treatment and apoptosis is a critical cellular process required for maintenance of tissue homeostasis. The Caspase family plays a critical role in apoptosis and caspase-8 is an initial caspase for the extrinsic apoptotic pathway. HECTD3, a new E3 ubiquitin ligase, was recently reported to confer breast cancer cells to cisplatin resistance. The possible mechanism is partially because HECTD3 promotes MALT1 ubiquitination and stabilization. (http://www.ncbi.nlm.nih.gov/pubmed/23358872)
However, the role and action mechanism of HECTD3 in cancer is still not completely understood.
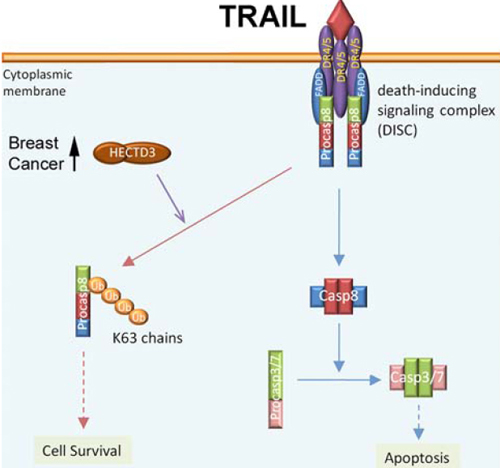
Dr. Ceshi Chen’s research group discovered a new pro-survival mechanism of HECTD3. The DOC domain of HECTD3 interacts with the DED domains of capsase-8; therefore, HECTD3 promotes capsase-8 ubiquitination through the non-degradative K63-linked polyubiquitin chains. The ubiquitination of caspase-8 by HECTD3 decreases the caspase-8 activity. Consistently, HECTD3 inhibits TRAIL, TNFa, and FasL induced caspase-8 activity-dependent cancer cell apoptosis. Importantly, HECTD3 is overexpressed in over 50% of breast carcinomas. These findings suggest that caspase-8 ubiquitination by HECTD3 promotes cancer cell survival and HECTD3 could be a therapeutic target to overcome drug resistance.
The work was published on Cell Death and Disease on Nov 28, 2013. This project was partially funded by the National Science Foundation of China and National Key Basic Research Program from Ministry of Science and Technology of China.
The full text is available online at: http://www.nature.com/cddis/journal/v4/n11/abs/cddis2013464a.html?src=email20130604CNS