Breast cancer is a common female malignant tumor. The triple negative breast cancer (TNBC), a type of breast cancer, lacks effective therapeutic targets. Patients are prone to recurrence and metastasis. Therefore, it is urgent to identify new therapeutic targets and develop effective drugs to improve the survival rate of patients with TNBC.
The previous studies have shown that transcription factor KLF5 is an effective therapeutic target for TNBC. Professor CHEN Ceshi from Tumor Biology group, Kunming Institute of Zoology, Chinese Academy of Sciences, Professor ZHOU Jia from the Department of Pharmacology and Toxicology, University of Texas Medical Branch, Galveston, Texas and Professor FENG Jing from the Department of Laboratory Medicine, Southern Medical University Affiliated Fengxian Hospital, Shanghai, collaborate to study a small molecule compound ML264 that can inhibit KLF5 in colorectal cancer.
According to the structure of ML264, researchers designed and synthesized a number of new derivatives. YD277 was identified as the most effective compound inhibiting the viability of TNBC cells in vitro. Further study found that YD277 significantly inhibited the proliferation of TNBC cells, induced cell cycle arrest in G1 / S phase and induced apoptosis.
Interestingly, YD277 functions independent on the inhibition of KLF5. YD277 significantly increased the expression of p21 and p27, the cleavage of Caspase-3, -7, -9 and PARP, and decreased the expression of Cyclin D1, Bcl2 and Bclxl. In terms of the functional mechanism, YD277 increased the expression of IRE1a, which resulted in the activation of endoplasmicreticulum stress signaling pathway. The anti-cancer effect of YD277 was greatly attenuated by blocking the IRE1a expression.
Finally, animal experiments showed that YD277 (15 mg / kg) effectively inhibited the xenograft growth in immunodeficient mice without obvious side effects.
In this study, YD277, a derivative derived from ML264, was first reported to activate the IRE1a signaling pathway, regulated the expression of several cell cycle and apoptosis-related proteins, inhibited the growth of human TNBC cells and induced apoptosis of cancer cells.
Two graduate students, CHEN Zekun from the Southern Medical University and WU Qiuju from Jinzhou Medical University, shared the first author. Prof. CHEN Ceshi, ZHOU Jia and FENG Jing shared the corresponding authors.
The research paper was published online in Theranostics: http://www.thno.org/v07p2339.htm#coraddress. This study was funded by the Chinese Academy of Sciences, the National Natural Science Foundation, and the NIH of United States.
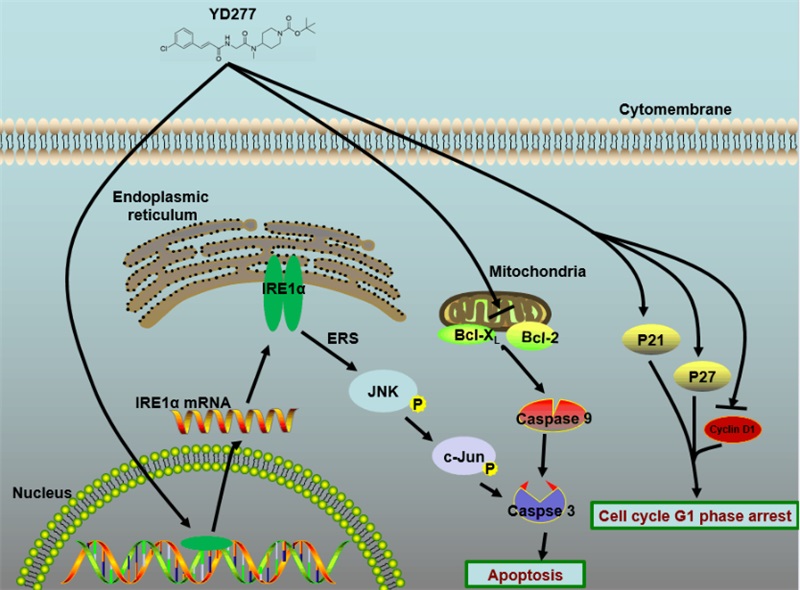
YD277 partially inhibits triple negative breast cancer by activating endoplasmic reticulum stress pathway (Image by CHEN Ceshi’s Group)
(By WU Qiuju; Editor: HE Linxi)
Contact:
CHEN Ceshi
chenc@mail.kiz.ac.cn
