Viviparity (live-bearing) is a reproductive mode in which pregnant females maintain developing embryos inside their reproductive tracts and give birth directly to offspring. In contrast, oviparity (egg-laying) is the reproductive pattern in which females lay eggs that continue to develop independently of the mother until hatching. Viviparous species provide an environment for embryonic development and protect the embryo from environmental threats.
It is an important basic biological question of how oviparity evolved to viviparity. Squamate reptiles, including lizards, snakes and amphisbaenians, offer an ideal model system to study the evolutionary transition from oviparity to viviparity in vertebrates.
About 20% of extant squamate species are viviparous. Viviparity has evolved between 98–129 times (depending on the analysis) in squamates, accounting for over two-thirds of all origins of viviparity in vertebrates. In addition, many of these origins of viviparity are evolutionarily recent, as viviparity has arisen in species or populations of otherwise oviparous clades.
Previous studies has confirmed that this transition in parity requires a number of physiological, morphological, and immunological changes, including reduced eggshell, delayed oviposition, placental development for supply of water and nutrition to the embryo by the mother, enhanced gas exchange, and suppression of maternal immune rejection of the embryo. However, the specific changes to genes or their expression that result in these major transformations are largely unknown.
A team of scientists from the Kunming Institute of Zoology of the Chinese Academy of Sciences (KIZ, CAS), together with another five teams from different institutes and universities, sequenced the genomes of viviparous Phrynocephalus vlangalii and oviparous P. przewalskii, and compared the transcriptomes of maternal oviducts through the development of eggs and embryos of both species (Fig. 1 & 2). In addition, they further compared the transcriptomes of three other pairs of closely related oviparous and viviparous species of the genera Eremias, Scincella, and Sphenomorphus to examine potential sequence convergence associated with viviparity across independent origins of this transition.
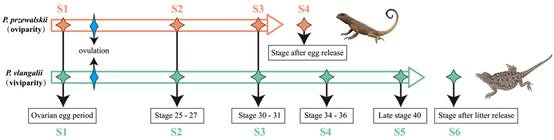
Fig. 1. Sample collection relative to the reproductive cycles. (Image by GAO Wei)

Fig. 2. Photos of P. vlangalii (A, Image by ZHOU Weiwei) and P. przewalskii (B, Image by QIN Yin); Field work photos of ZHOU Weiwei (C, Image by GAO Wei) and GAO Wei (D, Image by CHEN Yijing).
The comparative analysis of stage-specific highly expressed genes (HEGs) in S1, when an eggshell gland is expressed in egg-laying species, suggests a clear mechanism for eggshell degeneration in viviparous species (Fig. 3). Most of S1-specific HEGs in P. przewalskii (oviparous), which involved in uterine cell differentiation, proliferation and morphogenesis, gland development, were not highly expressed at S1 in viviparous P. vlangalii. And genes in estrogen receptor (ESR) pathway which were reported related to shell gland formation highly expressed at S1 in P. przewalskii, but reverse in viviparous species. These specific expression pattern changes appear to be among the changes associated with the loss of eggshell formation in the viviparous species.
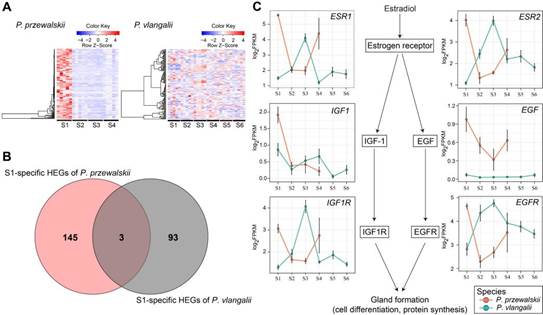
Fig. 3. Expression pattern of genes related to shell gland degeneration. (A) Expression heatmaps of genes specifically highly expressed at S1 in P. przewalskii (left) and orthologous genes in P. vlangalii (right). (B) Comparison of genes specific highly expressed before ovulation (at S1) of two species. (C) Expression pattern of key genes that are related the expression of shell glands. Red lines and circles represent gene-expression in P. przewalskii, green lines and circles represent gene-expression in P. vlangalii. Error bars present ±1 SE. (Image by GAO Wei)
Differential expression pattern were also found between two species at S3, which is soon before egg-laying in oviparous P. przewalskii. Estrogen receptor (ESR1) and other growth factor receptor (GHR and IGF1R) may play an important role in placenta development in viviparous species. And the down-regulation of PTGS2 at S4 and sustained highly expression of ADRB2 may promote the egg retention in viviparous P. vlangalii.
At last, four genes (C7, NKTR, NBEAL2, PTX2) were detected that experienced amino acid replacements at same position across four viviparous lizards. And they are all related to immune regulation. But this level was not significantly higher than by chance, which suggests that most of the changes that produce the oviparity–viviparity transition are changes in gene expression.
This work was published in PNAS journal (https://doi.org/10.1073/pnas.1816086116). GAO Wei, Dr. SUN Yanbo, Dr. ZHOU Weiwei, and XIONG Zijun are co-first authors of this work. Dr. CHE Jing, Dr. ZHANG Yaping, Dr. JI Xiangand Dr. HILLIS David are corresponding authors. This work was supported by grants from the Strategic Priority Research Program (B) Grant of the Chinese Academy of Sciences (CAS), the National Key Research and Development Program of China, National Natural Science Foundation of China Grant, the Animal Branch of the Germplasm Bank of Wild Species of CAS (the Large Research Infrastructure Funding), the Youth Innovation Promotion Association CAS, CAS “Light of West China” Program, and CAS President’s International Fellowship Initiative.
(By GAO Wei; Editor: HE Linxi)
Contact:
HE Linxi
helinxi@mail.kiz.ac.cn
