The study carried out with amphibians as models has made many outstanding original contributions to the life science process, such as muscle metabolism, chemical basis of nerve conduction, somatic cell cloning etc. The skin of amphibians is responsible for important physiological functions of breathing, water balance, mucosal barrier and immunity, but little is known about the mechanisms of cell biology and molecular biology.
Classic membrane receptors, ion channels and transports are directly transported to defined cellular membranes after synthesis in the cytoplasm. Pore- forming proteins (PFPs) are secreted as water-soluble proteins; once they reach their target cell membrane, these nonclassic membrane proteins can change their conformation extensively and convert to a transmembrane pore structure. The current understanding of PFPs is mainly focused on their cytotoxic effects, while little is known about their other cellular functions and action modes.
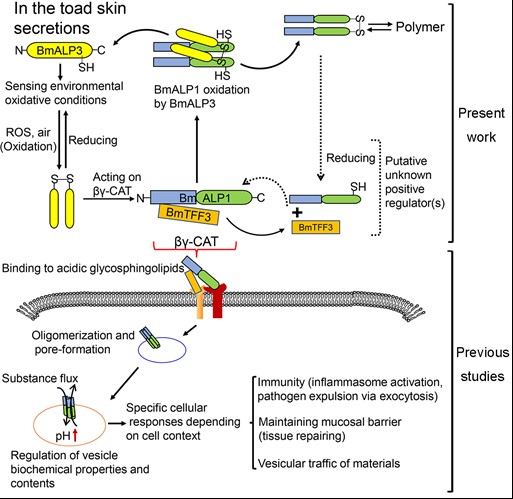
The Biotoxin and Human Diseases Research Group of Kunming Institute of Zoology, Chinese Academy of Sciences, led by researcher ZHANG Yun, identified the first vertebrate novel pore-forming protein and trefoil factor complex (CAT) fromBombina maxima (Invention Patent ZL200810058028.5; PLoS One, 2008). Take this protein machine as a model molecule, the research group has revealed a newly cell regulation strategy and molecular pathway after long-term research, that is, the secretory pore-forming protein acts on the endocytosis/ lysosomes to form a specific material channel, and then regulates the cell state and functions. Therefore, CAT also represents a class of secretory cell endocytosis channel proteins that are unknown so far. After assembly outside the cell, this channel protein (CAT) endocytosis via the double-receptor binding mode (Commun Biol. 2019). This action results in forming a membrane channel in the endocytosis/lysosome and then regulate its biochemical properties, including material exchange and pH, etc., which play an important role in the host innate immunity, mucosal barrier repair and cellular material transport (Proc Natl Acad Sci USA 2014, cover story; J Infect Dis 2017; FASEB J. 2019).
Recently, the Kunming Institute of Zoology and the University of Science and Technology of China have further revealed that the oxygen-dependent homologous protein regulation mechanism of the secretory cell endocytosis channel. The paralog of CAT, named BmALP3, was first identified. Though lack membrane pore-forming capacity, BmALP3 can sense environmental oxygen tension and transformed between monomer and homodimer, and then negatively regulates the assembly and biological activity of CAT via disulfide-bond exchange. BmALP3 is inactivated under hypoxic conditions, which resulting in the release of the inhibitory state of the effector protein machine CAT and promotes cell emergency and inflammatory response. Interestingly, the structural basis of oxygen-dependent regulation mode is well conserved in numerous animals and plants. The paper titled "A cellular endolysosome-modulating pore-forming protein from a toad is negatively regulated by its paralog under oxidizing conditions" has been published online in the Journal of Biological Chemistry (https://www.jbc.org/content/early/2020/06/04/jbc.RA120.013556).
The research not only further consolidates the biological significance and physiological role of the secretory cell endocytosis channel pathway, but also provides new ideas and clues for people to in-depth study of the cellular and molecular strategies and mechanisms of hypoxic stress and inflammation. What’s more, it has positive scientific significance on helping people understand the mechanism of human diseases and drug development. WANG Qiquan, BIAN Xianling and ZENG Lin of the Kunming Institute of Zoology, Chinese Academy of Sciences are the co-first authors of the article. ZHANG Yun, GUO Xiaolong of Kunming Institute of Zoology and LI Xu of the University of Science and Technology of China are the co-corresponding authors of the article. This research was financially supported by grants from the National Natural Science Foundation of China and the Yunling Scholar Program.
The regulation mode of oxygen-dependent secretory cell endocytosis channel
(Imaged by ZHANG Yun)
(By ZHANG Yun, Editor: YANG Yingrun)
Contact:
YANG Yinrun
yangyingrun@mail.kiz.ac.cn
