A research team led by Prof. ZHENG Ping from Kunming Institute of Zoology, Chinese Academy of Sciences, collaborating with the Prof. MENG Feilong from Shanghai Institute of Biochemistry and Cell Biology, Center for Excellence in Molecular Cell Science, provided the first proof of concept demonstrating the region-specific regulations of DSB formation and repair in subtypes of NSPCs. The finding was published in Science Advances on October 28, 2020.
Stem cells are the basis of organism development and tissue homeostasis, and genomic stability is prerequisite for stem cell maintenance and regenerative medicine application. Studying how stem cells maintain genomic stability can help advance the safe use of stem cells and understand the pathogenesis of related developmental diseases. The researchers have concerned about the unique mechanisms of stem cells maintain genomic stability. In the previous work, they identified that Filia regulates the genomic stability of embryonic stem cells, and revealed its different pathways(Cell Stem Cell 2015,16(6):684-698;Cell Research 2018,28(1):69-89),and also found that mutations of human homologous genes cause embryonic development failure and recurrent abortion(PloS Biology 2019,17(10): e3000468)。
Compared with embryonic stem cells, tissue stem cells have only specific developmental potential. But wether they have some common regulatory mechanisms for maintaining genomic stability? To investigate this problem, the researchers investigated the expression and function of Filia in neural stem/progenitor cells (NSPCs) of mice. Intriguingly, they found that Filia was expressed in cultured hippocampal NSPCs and responded to exogenous DNA damage. Here the researchers identified a regional regulator Filia, which is predominantly expressed in mouse hippocampal NSPCs after birth, and regulates DNA double strand break (DSB) formation. Filia-/- mice had impaired hippocampal NSPCs proliferation and neurogenesis. In addition, the complexity of dendrites in neurons were notably decreased in Filia-/- mice. Consequently, Filia-/- mice were deficient in learning, memory, and mood regulations. To further understand how genomic instability leads to neurogenesis and dysfunction, they performed high-throughput genome-wide translocation sequencing (HTGTS) to map the genome-wide DSBs. The results showed that the density of DSBs hotspots were notably higher in Filia-/- NSPCs. Moreover, 65 genes (>80kb) including 16 RDCs genes were affected by Filia and displayed increased DSBs density in Filia-/- NSPCs under normal culture condition. GO enrichment analysis revealed that these genes were involved in regulation of neurogenesis, such as cell adhesion, neuronal migration and synaptic assembly. Notably, the positions of splicing in several RDCs genes overlapped with the positions of DSBs, suggesting that the replication stress-induced DSBs at RDCs genes might be able to affect splicing.
In summary, this study revealed that Filia specifically regulates genomic stability and neurogenesis of hippocampal NSPCs, and provided the first proof of concept demonstrating the region specific regulations of DSBs formation and repair in hippocampal NSPCs.
This study, entitled “Genome integrity and neurogenesis of postnatal hippocampal neural stem/progenitor cells require a unique regulator Filia”, was published in Science Advances on October 28, 2020. Web link: https://advances.sciencemag.org/content/6/44/eaba0682 . Dr. ZHENG Ping and Dr. MENG Feilong are the co-corresponding authors, and LI Jingzheng (PH.D student) is the first author in this paper. This study was financially supported by grants from the National Key Research and Development Program of China, Stem Cell and Translational Research (2016YFA0100300), the Chinese NSFC grant to P.Z. (31930027), the exchange program of State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences (GREKF20-15), and the Basic Research Project of Yunnan Province to L. W. (2018FB056).
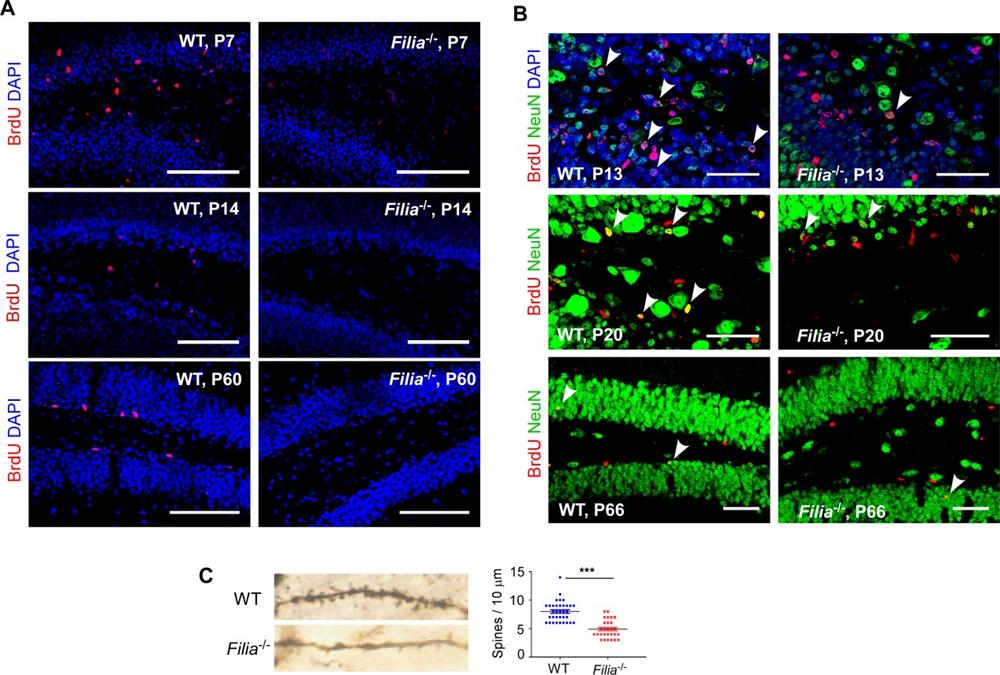
Filia deletion impairs hippocampal NSPCs proliferation and neurogenesis.
(Image by LI Jingzheng, copyright preserved)
(By LI Jingzheng, Editor: YANG Yingrun)
Contact:
YANG Yingrun
yangyingrun@mail.kiz.ac.cn
