Increasing evidence suggests that interspecific hybridization is crucial to speciation. However, chromatin incompatibility during interspecific hybridization often renders this process. Genomic imbalances such as chromosomal DNA loss and rearrangements leading to infertility have been commonly noted in hybrids. The mechanism underlying reproductive isolation of interspecific hybridization remains elusive.
To understand the genetic and regulatory basis of genomic imbalances leading to infertility, a joint research team from The Chinese University of Hong Kong and Kunming Institute of Zoology of the Chinese Academy of Sciences has investigated the maternal factors that regulate reproductive isolation in Xenopus hybrids, and suggest that maternally defined H3K4me3 is involved in establishing reproductive isolation and balances the different subgenomes in hybrid embryos of Xenopus.
Researchers assessed genetic and epigenetic alterations that regulate reproductive isolation between the two types of hybrids as te×ls (X. tropicalis eggs crossed with X. laevis sperm) with developmental arrest and viable le×ts (X. laevis egg with X. tropicalis sperm) and found that Chromosomal rearrangement or chromatin incompatibility is commonly observed in interspecific hybridization.
Then, transcriptomics highlighted that the P53 pathway was overactivated, and the Wnt signaling pathway was suppressed in te×ls hybrids. Moreover, the lack of maternal H3K4me3 in te×ls disturbed the balance of gene expression between the L and S subgenomes in this hybrid. The lack of H3K4me3 in te×ls hybrids may induce a failure in zygotic genome activation (ZGA) activation, which may also contribute for the development failure. The authors also found that H3K4me3 regulated the expression of Mdm2 gene and inhibited the activation of P53 signaling pathway, attenuation of p53 can postpone the arrested development of te×ls. The results indicated that incompatibility among subgenomes that resulted from the dysregulation of maternally defined H3K4me3, at least in part, caused embryonic lethality in the te×ls hybrids.
Taken together, this study firstly reveals the importance of maternally defined H3K4me3 modifications in reproductive isolation and in maintaining the subgenome in hybrids and sheds light on the effects of maternally defined epigenetic modifications on embryonic development. Studying the Xenopus hybrids and deciphering the mechanisms underlying speciation will expand our understanding of reproductive isolation as well as DNA repair and epigenetic modifications, which regulate early embryonic development.
This study, entitled “Modification of maternally defined H3K4me3 regulates the inviability of interspecific Xenopus hybrids” was published in Science Advance. Prof. LU Xuemei and Prof. ZHAO Hui are the co-corresponding authors. LONG Qi and YAN Kai are the co-first authors.
This project was supported by the National Natural Science Foundation of China, National Key Research and Development Program of China Stem Cell and Translational Research, Strategic Priority Research Program of the Chinese Academy of Sciences, Research Grants Council of Hong Kong, CAS "Light of West China" Program and the Yunnan Key Laboratory of Biodiversity Information and Guangdong Province Science and Technology Program Award.
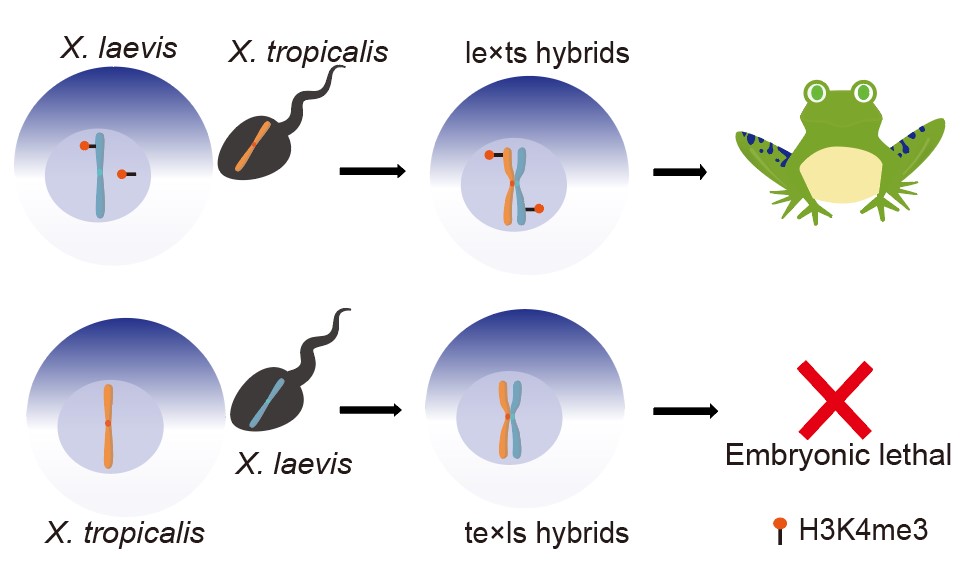
Schematic diagram illustrating that maternally defined H3K4me3 modifications regulate reproductive isolation in X. laevis and X. tropicalis, resulting in different fates of the two hybrids. (Image by Prof. Lu’s group of KIZ)
'
Contact: LU Xuemei, Principal Investigator
Kunming Institute of Zoology, Chinese Academy of Sciences,
Kunming, Yunnan 650201, China
Tel: 0871-65199961
E- mail: xuemeilu@mail.kiz.ac.cn
